EFFICACY OF COMBINATION OF MIDODRINE WITH WEEKLY ALBUMIN INFUSION VERSUS STANDARD MEDICAL THERAPY IN DECOMPENSATED LIVER CIRRHOSIS WITH REFRACTORY ASCITES
Introduction
Patients with cirrhosis have a net extracellular fluid overload and a central effective circulating hypovolemia. The resulting neurohumoral compensatory response favours the accumulation of fluids into the peritoneal cavity (ascites), lower limbs (edema) or occasionally all over the body (generalised anasarca). The deranged systemic hemodynamics (hyperdynamic circulatory syndrome) is characterized by elevated cardiac output with decrease in systemic vascular resistance and lowering of blood pressure1. In cirrhosis, due to sinusoidal portal hypertension, there is consequent nitric oxide induced splanchnic vasodilation. There is also reduced systemic vascular resistance in cirrhotics. This leads to reduced effective arterial blood volume. This compounded with hyperdynamic circulation, results in neurohormonal systems activation namely renin-angiotensin- aldosterone system2. During the initial phases of decompensated cirrhosis, when the activation of vasoconstrictor systems is moderate, patients develop sodium retention and ascites. In subsequent stages, activation of anti-diuretic hormone leads to dilutional hyponatremia. Finally, in the most advanced phase, when circulatory dysfunction is extreme, the renal vasodilatory systems are overcome and patients develop severe renal vasoconstriction and Type 2 hepato-renal syndrome (HRS)3. Few can progress into Type 1 HRS especially in presence of bacterial infection such as spontaneous bacterial peritonitis4. The median survival in Type 1 and Type 2 HRS is a dismal 1 month and 6 months respectively3. The annual risk of type 1 HRS development in patients with decompensated cirrhosis is about 20%, and within 5 years, it increases to 40%5.
Hemodynamic changes in cirrhosis6
Compensated cirrhosis: Increased cardiac output, normal heart rate and normal mean arterial pressure, normal effective arterial blood volume and a normal GFR
Decompensated cirrhosis: Increased cardiac output, increased heart rate, decreased mean arterial pressure, reduced effective arterial blood volume and finally reduced GFR
The long term recommended curative option for decompensated liver cirrhosis with refractory ascites going into Type 2 HRS is liver transplantation. A large proportion of cirrhotic patients succumb while waiting on the transplant list. Hence, there is a large unmet need for studying newer treatment modalities, which can prolong survival while on the waiting list.
Reduced albumin concentration is one of the hallmarks of decompensated cirrhosis due to poor liver synthetic functions. This results in reduced intra-oncotic pressure, leading to third space loss and consequent ascites and fluid overload. By giving albumin, this intra-oncotic pressure is improved, thereby helping in improving the effective arterial blood volume, leading to improved renal perfusion and consequent reversal of HRS. This is further enhanced by combining with terlipressin which will cause splanchnic vasoconstriction and improve renal blood flow, hence reversing type 1 HRS in 34 – 65% patients7. However the recurrence of HRS is seen in 15 - 22% patients after treatment withdrawal.8 A European multicenter, randomized, controlled trial of terlipressin and albumin vs albumin monotherapy in 46 patients with both types of HRS demonstrated an improvement in renal function but no survival advantage at 3 months9. Hence, liver transplantation is still the optimal therapy for HRS, and use of terlipressin is considered as a bridge to transplantation.10
Albumin is a multifunctional protein11. By albumin supplementation, most importantly there is improvement in the intra-oncotic pressure. It also is a major antioxidant and helps in free radical scavenging. Thirdly, it has immunomodulatory role in SIRS. There is interesting data that albumin has ionotropic effect demonstrated in rat models12. In the recently published landmark ANSWER trial13, cirrhotics with uncomplicated ascites who were treated with anti-aldosteronic drugs (≥200 mg/day) and furosemide (≥25 mg/day), were randomly assigned to receive either standard medical treatment (SMT) or SMT plus HA (40 g twice weekly for 2 weeks, and then 40 g weekly) for up to 18 months. Overall 18-month survival was significantly higher in the SMT plus HA than in the SMT group (77% vs 66%; p - 0•028), resulting in a 38% reduction in the mortality hazard ratio (0•62 [95% CI 0•40–0•95]). However, the median MELD score in both groups were 13 and 12, implying that these patients were not so sick for transplant and were in relatively in well preserved function with good hemodynamics.
Midodrine is an oral alpha-1 adrenergic agonist, metabolised in the liver into active metabolite desglymidodrine, with plasma half-life of 25 minutes14. It improves the mean arterial pressure, leading to improved renal plasma flow, improved GFR, translating into reduced RAAS activation and improved renal hemodynamics and ascites mobilisation15. Another study16 had shown that with short-term administration of midodrine for 7 days, was associated with significant increase in urine sodium excretion in patients without and with ascites, in line with significant increases in mean arterial pressure and systemic vascular resistance, and significant decreases in cardiac output and heart rate. Significant increases in glomerular filtration rate, filtration fraction, and urine volume and significant decreases in plasma renin activity and aldosterone were observed in patients with ascites. Small randomised controlled trials17,18 have shown that midodrine improves ascites mobilisation, hyponatremia to some extent and short term mortality benefit in refractory ascites. In another recently published study19 comparing midodrine versus albumin infusion in refractory ascites undergoing large volume paracentesis, there was no significant difference noted between the groups in the development of renal impairment, hyponatremia, or mortality 6 and 30 days after LVP. However, a significant increase in 24-h urine sodium excretion was noted in the midodrine 30-day group.
Rationale
Albumin will improve the blood volume expansion which will dampen the effect of neurohormonal vasoconstrictor systems and improve the renal perfusion. Midodrine being an oral alpha 1-adrenergic agonist will improve the systemic vascular resistance resulting in improved mean arterial pressure, better systemic and renal hemodynamics and hence improve natriuresis and ascites mobilisation. Combination of weekly albumin infusion with oral midodrine should theoretically improve the systemic and renal hemodynamics and consequently translate to overall survival, reduced need for large volume paracentesis, other complications of portal hypertension in refractory ascites.
Hypothesis
Combination of once weekly albumin infusion with oral midodrine is better than standard medical therapy alone in improving survival in a patient of decompensated liver cirrhosis with refractory ascites
Study Objectives
- To study whether combination of once weekly albumin infusion with oral midodrine is better than standard medical therapy alone in improving survival in a patient of decompensated liver cirrhosis with refractory or recurrent ascites.
- To study whether combination of once weekly albumin infusion with oral midodrine is better than standard medical therapy alone in improving renal functions in patients and reduce the incidence of acute kidney injury including hepatorenal syndrome
- To study whether combination of once weekly albumin infusion with oral midodrine is better than standard medical therapy alone in improving ascites mobilisation, and reducing the need for large volume paracentesis.
- To study whether combination of once weekly albumin infusion with oral midodrine is better than standard medical therapy alone in improving the mean arterial pressure, renal hemodynamics.
- To study whether combination of once weekly albumin infusion with oral midodrine is better than standard medical therapy alone in improving quality of life in decompensated liver cirrhosis.
Definitions
- Uncomplicated ascites- not infected and not associated with HRS
- Refractory ascites - cannot be mobilised or early recurrence of which (that is after therapeutic paracentesis) cannot be prevented by medical treatment
- Diuretic resistant ascites - refractory to dietary salt restriction and intensive diuretic treatment [spironolactone 400mg and frusemide 160mg per day and salt restricted diet of less than 90mmol/day (5.2g/day)]
- Diuretic intolerant ascites - refractory to therapy due to the development of diuretic induced complications
- Prerequisites for diagnoses
- Treatment duration: Intensive diuretic therapy [spironolactone 400mg and frusemide 160mg per day and salt restricted diet of less than 90mmol/day (5.2g/day)] for atleast one week
- Lack of response: Mean weight loss of < 0.8 kg over 4 days & urinary sodium output less than the sodium intake.
- Early ascites recurrence: Re-appearance of grade 2 or 3 ascites within 4 weeks of initial mobilization.
- Diuretic induced complications such as hepatic encephalopathy or new renal impairment with rise in serum creatinine to 0.3mg/dl in 24 hours from baseline.
Criteria for hepatorenal syndrome
Major criteria
- Chronic liver disease with advanced hepatic failure and portal hypertension
- Serum creatinine >1.5 mg/dL or doubling of creatinine or rise by more than 0.3mg/dl in 24 hours, reflecting decreased glomerular infiltration rate
- Absence of shock, bacterial infection, and current or recent treatment with nephrotoxic drugs; absence of gastrointestinal or renal fluid losses
- No sustained improvement in renal function (decrease in serum creatinine to (1.5 mg/dL) after diuretic withdrawal and plasma volume expansion with intravenous albumin (1 g/kg body weight up to a maximum of 100 g)
- Proteinuria < 500 mg/dL and no evidence of parenchymal renal disease by urinalysis, or of obstructive uropathy by ultrasonography
Minor criteria
- Urine volume < 500 mL/24 h
- Urine sodium <10 mEq/L
- Urine osmolality greater than plasma osmolality
- Urine red blood cells < 50 per high power field
- Serum sodium <130 mEq/L
Tests to check renal hemodynamics
- Urine sodium excretion
- Plasma renin activity
- Fractional excretion of sodium (%)
- eGFR estimation
Relative Contraindications to oral midodrine administration
- Coronary artery disease
- Cardiomyopathies
- Cardiac arrhythmias
- Cardiac or respiratory failure
- Arterial hypertension
- Cerebrovascular disease
- Peripheral vascular disease
- Bronchospasm, asthma
- Advanced hepatocellular carcinoma
Relative contraindications to albumin administration
- Poor cardiac function due to coronary artery disease/ cardiomyopathies/ cardiac arrhythmias
- Pulmonary edema
- Fluid overload state with generalised anasarca
- Previous history of allergic reactions to albumin administration
- Advanced hepatocellular carcinoma
Standard medical therapy
- Fluid restriction to less than 1 litre/day
- Salt restriction to less than 4gm/day
- Periodic large volume paracentesis as and when required due to overt abdominal distension or respiratory distress.
- During large volume paracentesis, patients will be given albumin infusion at dose of 8g / litre of fluid tapped with 50% of the dose given during paracentesis and the other 50% 6 hours later for adequate volume replacement
- Non selective beta blockers will be stopped
- Upper GI endoscopy yearly once for small varices and every six months once for large varices after variceal eradication by endoscopic variceal ligation
- Six monthly once alpha fetoprotein and USG abdomen for HCC surveillance
- Diuretics (furosemide 40mg/day and spironolactone 100mg/day) to be started and closely monitored for acute kidney injury before further increments in dosage, to achieve mean weight loss of > 0.8 kg over 4 days & urinary sodium output more than the sodium intake. If there is acute kidney injury, then diuretics will be stopped and plasma volume expansion with intravenous albumin (1 g/kg body weight).
Materials & Methods
Inclusion criteria
- All cirrhotic (sinusoidal portal hypertension) patients, age above 18 years, either gender, irrespective of aetiology without acute kidney injury
- Refractory ascites
Exclusion criteria
Patients with
- cardiac conditions such as ischemic heart disease, congestive cardiac failure
- hepatic encephalopathy
- active sepsis such as spontaneous bacterial peritonitis or blood culture positivity
- tuberculous ascites
- chronic kidney disease
- active malignancy
- any contraindications to oral midodrine administration as listed above
- any contraindications to intravenous albumin administration as listed above
- not willing to participate in the study
Baseline clinical assessment
- Dietary compliance with salt and water restriction per day
- Corrected body weight
- Heart rate and Mean arterial pressure
- Previous history of large volume paracentesis (LVP), frequency of LVP, average amount of fluid drained during each LVP
- Previous history of other complications of portal hypertension namely hepatic encephalopathy, acute kidney injury, variceal bleed, spontaneous bacterial peritonitis
- Previous history of ICU admissions for complications of portal hypertension
- Impact on quality of life due to liver cirrhosis assessment by using CLD questionnaire23
Investigations
- Hematology
- CBC, Prothrombin time and INR
- Biochemistry
- Liver function test
- Kidney function test
- Serum electrolytes
- Plasma renin activity
- Urine analysis
- Urine routine for protein, RBC sediment, pus cells
- Urine spot sodium
- Urine protein – creatinine ratio
- eGFR
- Etiology of chronic liver disease (if not available)
- Infectious etiology: HBsAg, total anti HBc, anti HCV
- Non-infectious etiology: Autoimmune markers, copper studies, iron studies, HOMA IR, FBS
- Sepsis screening (if indicated)
- Blood culture
- Urine culture
- Chest X ray
- Ascitic fluid analysis (if indicated)
- UGI endoscopy (if not done within past 6 months)
- Imaging (if not available)
- USG abdomen with Doppler for spleno-portal axis ‘or’
- CECT- Triple phase upper abdomen
- 2 D Echo
- RA/ RV dilatation
- PA pressure
- IVC dilatation
- Ejection fraction
METHODOLOGY
All patients enrolled in the study, will be admitted and started on standard medical therapy as mentioned previously. An informed consent will be obtained.
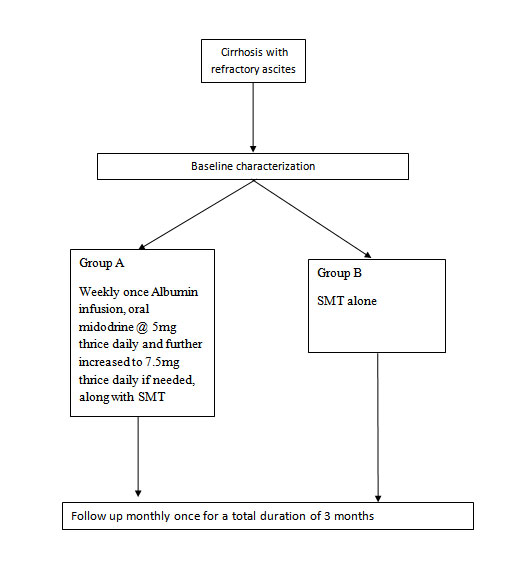
- Patients will be randomised into Group A or Group B as per block randomisation chart (computer generated)
- Weekly once intravenous Recombinant Human Albumin 20% 100ml administration slowly as an infusion over 4 -5 hours with heart rate and oxygen saturation monitoring. Oral midodrine will be started at 5mg thrice daily and increased to 7.5mg thrice daily in order to maintain the mean arterial pressure is more than 65mm Hg. Standard medical therapy will be continued as mentioned previously.
- Standard medical therapy alone will be given
- Study duration: Three months
- Daily monitoring of following parameters:
- Intake output
- Body Weight
- Monthly once follow up with following parameters
- Routine labs namely liver function test, PT/INR, kidney function test including serum electrolytes, complete blood count
- Urine sodium excretion and FeNa %
- eGFR estimation
- Plasma renin activity
- Heart rate and mean arterial pressure
- Impact on quality of life due to liver cirrhosis assessment by using CLD questionnaire23
- If any patient develops acute kidney injury during the study period, diuretics and any other nephrotoxic agents will be stopped and if required, aetiology for acute kidney injury will be evaluated
- If its prerenal due to diuretics or dehydration or fluid losses, then diuretics will be stopped furosemide will be stopped immediately and plasma volume expansion with intravenous albumin (1 g/kg body weight) will be given.
- If its hepato-renal syndrome, then recommended therapeutic combination of intravenous albumin administration along with terlipressin infusion will be started
- If its due to sepsis, then antibiotics will be started as per local infectious disease protocol or as per cultures if any are positive.
- Nephrology consultation will be sought and acute kidney injury closely monitored and managed. Renal replacement therapy will also be considered in special situations as per nephrologist advice.
- If any patient develops any complication of portal hypertension namely hepatic encephalopathy, variceal bleed, spontaneous bacterial peritonitis, then the patient will be temporarily censored for the survival analysis and managed as per standard of care.
Outcomes
- Primary
- Reduction in need for large volume paracentesis (LVP)
- Secondary
- Overall survival at three months
- Improvement in systemic and renal hemodynamics
- Incidence of new onset acute kidney injury
- Reduction in liver disease severity scores
- Reduction in need for large volume paracentesis (LVP)
- Development of new onset complications such as sepsis, variceal bleed, hepatic encephalopathy
- Improvement in quality of life by CLD questionnaire
- Safety of treatment.
Sample size calculation:
Study Design:
REFERENCES
- Gines P, Guevara M. Hyponatremia in cirrhosis: Pathogenesis, Clinical significance and management. Hepatology 2008; 48 (3): 1002- 1010
- Arroyo V and Fernandez J. Nat Rev Nephrol 2011; 7(9): 517-526
- Hartleb M and Gutkowski K. Kidneys in chronic liver diseases. World J Gastroenterol 2012; 18 (24): 3035- 49
- Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med 1999; 341: 403-409
- Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology 1993; 105: 229-236
- Ruiz-del-Arbol L, Urman J, Fernández J, González M, Navasa M et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology 2003; 38: 1210-1218
- Moreau R, Durand F, Poynard T, Duhamel C, Cervoni JP et al. Terlipressin in patients with cirrhosis and type 1 hepatorenal syndrome: a retrospective multicenter study. Gastroenterology 2002; 122: 923-930
- Gluud LL, Christensen K, Christensen E, Krag A. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology 2010; 51: 576-584
- Romanelli RG, La Villa G, Barletta G, Vizzutti F, Lanini F et al. Long-term albumin infusion improves survival in patients with cirrhosis and ascites: an unblinded randomized trial. World J Gastroenterol 2006; 12: 1403-1407
- Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009; 49: 2087-2107
- Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: Pathophysiologic basis of its role in treatment of cirrhosis and its complications. Hepatology 2013; 58 (5): 1836- 46
- Bortoluzzi A, Ceolotto G, Gola E, Sticca A, Bova S et al. Positive cardiac ionotropic effect of albumin infusion in rodents with cirrhosis and ascites: molecular mechanisms. Hepatology 2013: 57 (1): 266 – 76
- Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S et al. Long term albumin administration in decompensated cirrhosis (ANSWER): an open label randomised trial. Lancet 2018; 391: 2417 - 29
- Ali A, Farid S, Amin M, Kassem M, Al-Garem N, Al-Ghobashy M. Comparative Clinical Pharmacokinetics of Midodrine and Its Active Metabolite Desglymidodrine in Cirrhotic Patients with Tense Ascites Versus Healthy Volunteers. Clin Drug Investig 2016: 36 (2): 147 - 55
- Angeli P, Volpin R, Provan D, Bortoluzzi A, Craighero R et al. Acute effects of the oral administration of midodrine, an alpha adrenergic agonist, on renal hemodynamics and renal function in cirrhotic patients with ascites. Hepatology 1998; 28 (4): 937 - 43
- Kalambokis G, Fotopoulos A, Economou A, Pappas K, Tsianos EV. Effects of a 7-day treatment with midodrine in non-azotemic cirrhotic patients with and without ascites. J Hepatol 2007; 46 (2): 213 – 21
- Singh V, Dhungana P, Singh B, Vijayverghia R, Nain CK et al. Midodrine in patients with cirrhosis and refractory or recurrent ascites- a randomised pilot study. J Hepatol 2012; 56 (2): 348 – 54
- Rai N, Singh B, Singh A, Vijayverghia R, Sharma N, Bhalla A, Singh V. Midodrine and Tolvaptan in patients with cirrhosis and refractory or recurrent ascites – a randomised pilot study. Liver Int 2017; 37 (3): 406 – 414
- Yosry A, Soliman ZA, Eletreby R, Hamza I, Ismail A, Elkady MA. Oral midodrine is comparable to albumin infusion in cirrhotic patients with refractory ascites undergoing large volume paracentesis: results of a pilot study. Eur J Gastroenterol Hepatol 2019; 31 (3): 345- 51
- Gines P, Angeli P, Lenz K, Moller S, Moore K et al. EASL clinical practice guidelines on management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome in cirrhosis. J Hepatol 2010; 53: 397 – 417
- Munoz JS. Hepatorenal syndrome. Med Clin N Am 2008; 92: 813 - 837
- European association for study of liver. EASL Clinical practice guidelines for management of patients with decompensated cirrhosis. J Hepatol 2018; 69 (2): 406 - 460
- Hanchanale P, Janani K, Varghese J, Vijaya S, Venkataraman J, Rela M. Quality of life in chronic liver disease patients using CLDQ questionnaire. J Clin Exp Hepatol 2015; 5: S44
| MIDODRINE + ALBUMIN PROTOCOL PROFORMA |
| PATIENT NAME: |
UHID: |
| AGE/SEX: |
|
| DIAGNOSIS: |
|
| |
WEIGHT: |
| BMI: |
|
| ETIOLOGY: |
|
| CO - MORBID STATUS |
|
|
CLINICAL STATUS
|
|
ASCITES
|
|
|
ASCITES & EDEMA
|
|
|
ANASARCA
|
|
Past History- (Y/N, with dates)
|
Viral hepatitis
|
|
|
Prior surgery/Blood transfusions
|
|
|
Diabetes mellitus
|
|
|
Tuberculosis
|
|
|
Drugs: NSAIDS/ Alternative medications / Herbal remedies
|
|
|
Antivirals or antitubercular drugs
|
|
H/O of alcohol -
Age of onset (Yr.) ………………………………
Total duration (yr.) ……………………………
Average quantity (g/day)-………………………
Last intake………………………………………
Type of Drink ………………………………….
Clinical Assessment:
| Parameter |
Baseline |
Month 1 |
Month 2 |
Month 3 |
Month 4 |
Month 5 |
Month 6 |
| Body weight (corrected) |
|
|
|
|
|
|
|
| Heart rate |
|
|
|
|
|
|
|
| Mean arterial pressure |
|
|
|
|
|
|
|
| CLD Q |
|
|
|
|
|
|
|
| Complications of PHT |
|
|
|
|
|
|
|
BASELINE LABS:
| Hb |
|
Total Bil |
|
| Total count |
|
Direct Bil |
|
| Neutrophils |
|
AST |
|
| Lymphocytes |
|
ALT |
|
| Platelet count |
|
ALP |
|
| INR |
|
GGT |
|
| Urea |
|
T. Protein |
|
| Creatinine |
|
Albumin |
|
| Sodium |
|
Potassium |
|
| Urine spot sodium |
|
Urine PCR |
|
| FeNa |
|
Plasma renin activity |
|
| CTP score |
|
MELD score |
|
Etiology of liver disease (if required) :
| Date |
|
| Total antiHBc |
|
| HBV DNA (if required) |
|
| Anti HCV |
|
| HCV RNA (if required) |
|
| ANA (1: 80 ) dilution |
|
| SMA( 1: 40) dilution |
|
| IgG |
|
| Ceruloplasmin |
|
| 24hour urine copper |
|
| Serum copper |
|
| Iron |
|
| TIBC |
|
| Transferrin Saturation |
|
| Ferritin |
|
| Ascitic fluid (if required) |
Date |
| Colour |
|
| T.Pr/Alb |
|
| SAAG |
|
| TLC |
|
| DLC |
|
| Gram Stain |
|
| Culture |
|
Urine analysis
Routine (at baseline)
| pH |
|
Protein |
|
| Protein |
|
RBCs |
|
| Sugar |
|
Pus cells |
|
| Urine Na: |
Urine RPC: |
| BaseLine Echo: |
|
Sepsis screening (if suspicion present)
| Blood culture |
|
| Urine culture |
|
| Chest X ray |
|
TREATMENT:
- Group A: Weekly albumin infusion + oral midodrine + SMT
- Group B: SMT alone
Monthly Follow up:
| Parameter |
Baseline |
Month 1 |
Month 2 |
Month 3 |
Month 4 |
Month 5 |
Month 6 |
| Hb |
|
|
|
|
|
|
|
| TLC |
|
|
|
|
|
|
|
| Platelet count |
|
|
|
|
|
|
|
| BUN |
|
|
|
|
|
|
|
| Creatinine |
|
|
|
|
|
|
|
| K+ |
|
|
|
|
|
|
|
| Na+ |
|
|
|
|
|
|
|
| eGFR |
|
|
|
|
|
|
|
| T. Bil |
|
|
|
|
|
|
|
| D. Bil |
|
|
|
|
|
|
|
| AST |
|
|
|
|
|
|
|
| ALT |
|
|
|
|
|
|
|
| ALP |
|
|
|
|
|
|
|
| GGT |
|
|
|
|
|
|
|
| T. protein |
|
|
|
|
|
|
|
| Albumin |
|
|
|
|
|
|
|
| INR |
|
|
|
|
|
|
|
| CTP score |
|
|
|
|
|
|
|
| MELD score |
|
|
|
|
|
|
|
| Urine sodium excretion |
|
|
|
|
|
|
|
| eGFR |
|
|
|
|
|
|
|
| Fe Na (%) |
|
|
|
|
|
|
|
| PRA levels |
|
|
|
|
|
|
|
Stoppage of treatment:
Adverse effects:
Renal replacement therapy:
New onset complications:
